Gail A. Cornwall, Ph.D.
Professor

Ph.D. Reproductive Biology
Johns Hopkins Bloomberg School of Public Health
Curriculum Vitae
Department of Cell Biology and Biochemistry
Texas Tech University Health Sciences Center
3601 4th Street, Lubbock, TX 79430-6540
Office Phone: (806) 743-2687
gail.cornwall@ttuhsc.edu
Research Interests
Assembly and function of biological amyloids in the reproductive tract and brain,
neurodegeneration, sperm maturation and epididymal function.
Current Projects
We study the assembly/structure and function of biological amyloids in the mammalian reproductive tract and brain and if, and how, functional amyloids contribute to disease including neurodegeneration and the epigenetic inheritance of disease.
Amyloids are proteins that self-assemble into highly ordered cross-β and cross-α-rich structures. Although previously thought to be pathological entities that contribute to neurodegenerative disease and prionopathies, there is now considerable evidence, including work from our lab, showing that many amyloids carry out critical biological roles and are known as functional amyloid. For our studies, we use a variety of biochemical, cell biological and biophysical approaches using recombinant proteins, cell culture and gene knockout mouse models. Whenever possible we try to translate our work in rodent to human. Our lab currently has three main areas of research focus.
Amyloid function in the reproductive tract and brain
We demonstrated that an extracellular amyloid matrix surrounds the maturing sperm in the normal mouse epididymal lumen (Fig 1). This extracellular amyloid includes four members of the reproductive CRES (cystatin-related epididymal spermatogenic) subgroup of family 2 cystatins of cysteine protease inhibitors, indicating a complex functional amyloid structure. We model our studies of epididymal amyloid matrix assembly/structure and function on bacterial biofilms and identified common structural components including an amyloid infrastructure, extracellular nucleic acids, and complex polysaccharides. Further, the epididymal amyloid matrix and CRES amyloids have host defense functions and form distinct amyloid structures (matrix, films, fibrils) with distinct host defense functions (bacterial trapping vs killing) depending on the pathogenicity of the bacterial strain (Fig 2). These studies reveal a strong rationale for why nature has kept the amyloid fold since its shape-shifting properties provides a plasticity that is integral for epididymal amyloid matrix function, and likely also that of other biological structures
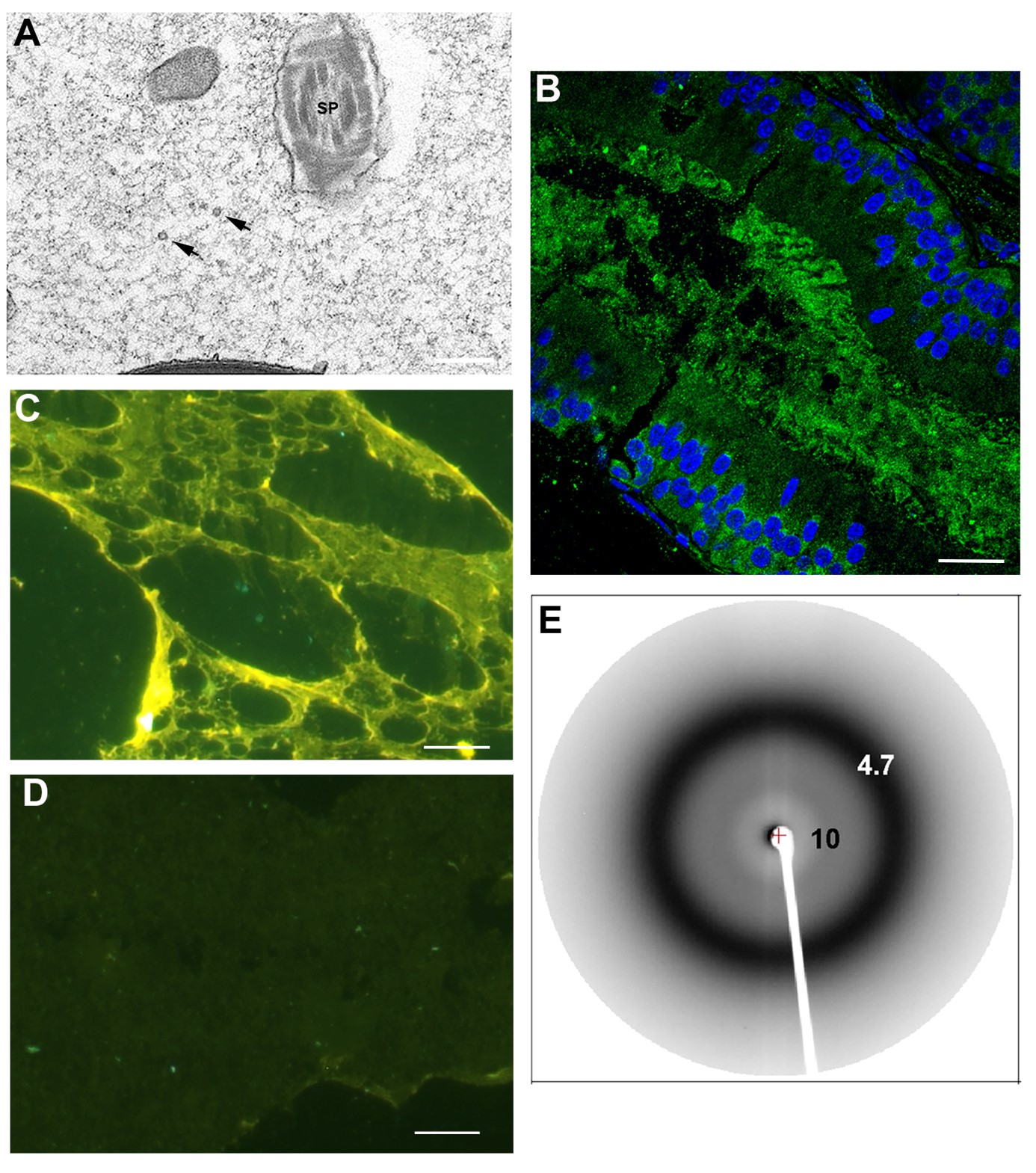 |
Figure 1. Amyloid matrix in the epididymal lumen. |
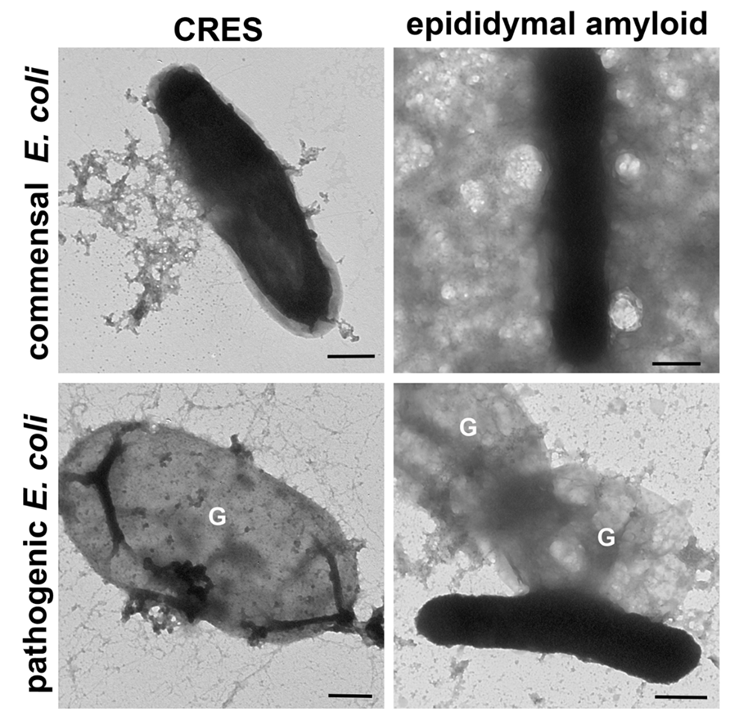 |
Figure 2. Pathogen-specific host defense responses of the CRES monomer and epididymal
amyloid matrix. |
Building on our studies in the epididymis, we are testing the hypothesis that a comparable extracellular amyloid matrix is a component of the mammalian brain and integral for synaptic plasticity. The brain extracellular matrix (ECM) plays critical roles in synaptic plasticity including experience/adaptive-based changes in brain function suggesting an inherent malleability in ECM structure. However, the underlying mechanisms that enable ECM plasticity are still unknown. Our early studies show CRES and other subgroup members are expressed by astrocytes and specific neuronal populations in the male and female mouse and human brain including the hippocampus and cortex. We are currently carrying out behavioral studies of the CRES KO, analyses to determine if CRES subgroup members are part of the brain ECM and their roles in brain function.
Molecular mechanisms of functional amyloid assembly.
Functional and pathological amyloids follow similar pathways during their assembly. Despite forming comparable structures, functional amyloids are distinct from pathological forms since they are produced in vivo without significant cytotoxicity and often reversibly disassemble. However, the mechanism(s) by which functional amyloids assemble and disassemble is poorly understood. With our collaborators R. Bryan Sutton (X-ray crystallographer, TTUHSC), Michael P. Latham (solution-state NMR spectroscopist, University of Minnesota) and Benjamin J. Wylie (solid-state NMR spectroscopist, TTU) we used CRES as a molecular model to follow its transition at the atomic level from monomer to advanced amyloid under conditions that mimic the in vivo environment. We discovered that CRES forms amyloid by two assembly mechanisms: classic cystatin domain swapping and a unique rigidification of a disulfide anchored flexible loop to β-strand (Fig 3). Using mutagenesis and structural and biophysical approaches we are now carrying out studies to determine the biological significance of the two assembly mechanisms on resulting CRES amyloid structure and function. We hypothesize the use of multiple assembly mechanisms may allow functional amyloids to adopt very different geometries if one pathway dominates over the other, which could be mediated by local environmental conditions. Further, the use of distinctive mechanisms to assemble amyloid within a common structure could also be a means to selectively disassemble specific β-sheet interactions allowing a highly dynamic and plastic functional amyloid structure.
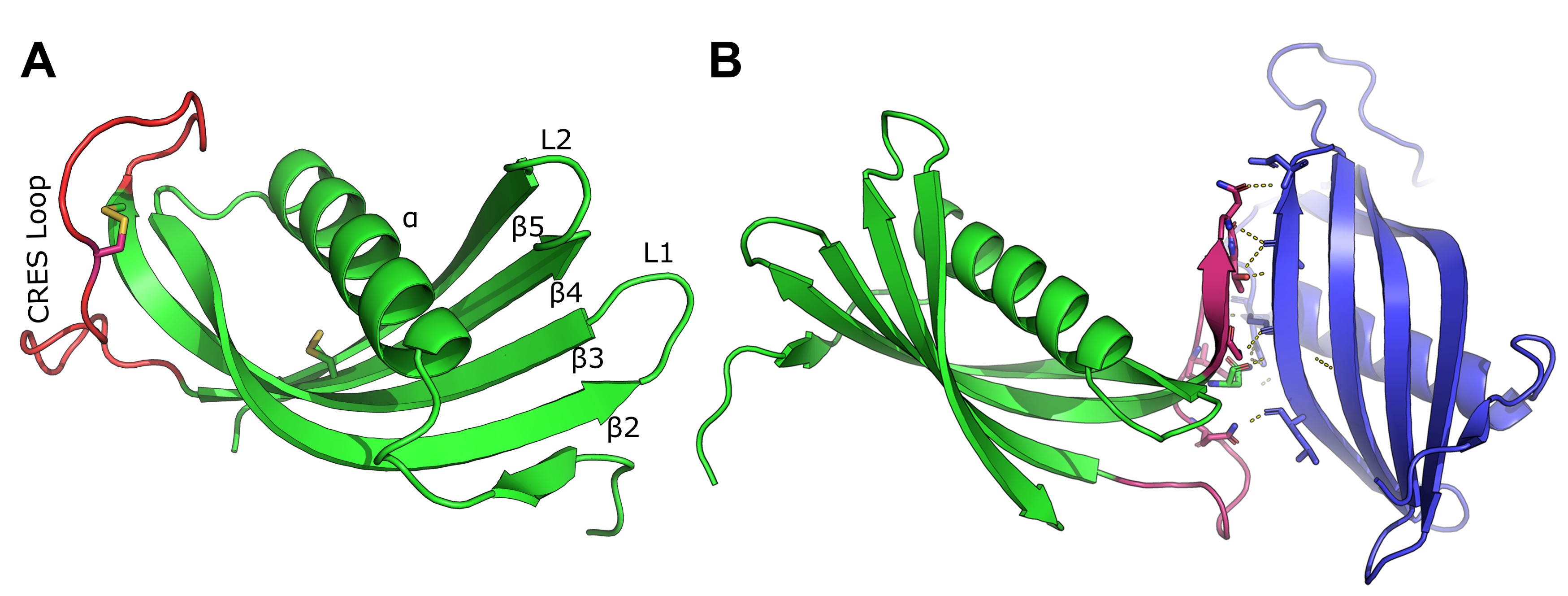 Figure 3. X-ray crystal structure of CRES.
Figure 3. X-ray crystal structure of CRES.
A) Ribbon diagram of the CRES monomer highlighting the CRES loop in red and the two
disulfide bonds as yellow sticks. B) Intermolecular interaction of two CRES molecules
in the crystallographic asymmetric unit. Highlighted in pink is the CRES loop and
β3 of one molecule forming a parallel β-sheet interaction with β5 of an adjacent molecule.
Hewetson et al., 2020.
Sperm amyloids - epigenetic carriers of inheritance.
Environmental exposures to the father can be transmitted to his offspring through the germline resulting in inherited phenotypes including disease such as obesity, diabetes, and cancer. Although changes in DNA methylation, histone modifications, and small RNAs have been observed, the epigenetic carrier in sperm is still unknown. Because of the significant impact on human health and accompanying economic consequences, it is critical that germline carrier(s) that respond to environmental stress and intergenerationally or transgenerationally transmit this information are identified. To answer this question we used an established rat model for transgenerational inheritance and discovered that in male germ cells two clinically relevant proteins involved in human disease, assembled into amyloid following exposure to the endocrine disruptor vinclozolin. Further, the amyloids correlated with a phenotype of germ cell apoptosis in the vinclozolin exposed males. Most importantly, germ cells from the sons and grandsons of the exposed males also contained amyloids despite their not being directly exposed to the environmental toxin. These results led us to hypothesize that amyloids may function as epigenetic carriers of inheritance. Although established in yeast, a prion-based mechanism of inheritance has not been demonstrated in higher organisms. In this project we are using the rat vinclozolin model to test our hypothesis. If successful our studies will result in conceptual advances towards answering the big questions of the role of the environment in the origins of disease, how some diseases are inherited, and how species evolve.
Selected Publications
- Myers C, Atkins GR, Villarreal J, Sutton RB, Cornwall GA. (2023). The Mouse Epididymal Amyloid Matrix: A Mammalian Counterpart of a Bacterial Biofilm. bioRxiv 2023.11.15.567275. doi: https://doi.org/10.1101/2023.11.15.567275
- Myers C and Cornwall GA. (2023). Host Defense Amyloids: Biosensors of the Immune System?, Andrology Nov 14. doi: 10.1111/andr.13555. PubMed
- Myers C, Hastert MC, and Cornwall GA. (2022). Host defense functions of the epididymal amyloid matrix. Mol Human Reprod 30;28(12):gaac038. doi: 10.1093/molehr/gaac038. PubMed
- Do HQ, Hewetson A, Borcik CG, Hastert MC, Whelly S, Wylie BJ, Sutton RB, Cornwall G.A. (2021). Cross-seeding between the functional amyloidogenic CRES and CRES3 family members and their regulation of Aβ assembly. J Biol Chem. Jan-Jun;296:100250. doi: 10.1074/jbc.RA120.015307. Epub 2021 Jan 9. PubMed
- Hewetson A, Khan NH, Dominguez MJ, Do HQ, Kusko RE, Borcik CG, Rigden DJ, Keegan RM, Sutton RB, Latham MP, Wylie BJ, Cornwall G.A. (2020). Maturation of the functional mouse CRES amyloid from globular form. Proc Natl Acad Sci U S A. Jul 14;117(28):16363-16372. doi: 10.1073/pnas.2006887117. Epub 2020 Jun 29. PubMed
- Do HQ, Hewetson A, Myers C, Khan NH, Hastert MC, M Harsini F, Latham MP, Wylie BJ,
Sutton RB, Cornwall G.A. (2019) The Functional Mammalian CRES (Cystatin-Related Epididymal Spermatogenic)
Amyloid Is Antiparallel β-Sheet Rich and Forms a Metastable Oligomer During Assembly.
Sci Rep. 2019 Jun 25;9(1):9210. doi: 10.1038/s41598-019-45545-w. PubMed
- Cornwall, G.A., Do HQ, Hewetson A, Muthusubramanian A, Myers C. (2019). The epididymal amyloid matrix: structure and putative functions. Andrology. Jan 20. doi: 10.1111/andr.12586. PubMed
- Hewetson A, Do HQ, Myers C, Muthusubramanian A, Sutton RB, Wylie BJ, Cornwall, G.A. (2017). Functional amyloids in reproduction. Biomolecules. 7(3). pii: E46. PubMed
- Whelly, S., Muthusubramanian A, Powell, J., Johnson, S., Hastert, M. C., and Cornwall, G.A. (2016). CRES (cystatin-related epididymal spermatogenic) subgroup members are part of an amyloid matrix and associated with extracellular vesicles in the mouse epididymal lumen. Mol Human Reprod. 22:729-744. PubMed
- Egge, N., Muthusubramanian A, and Cornwall, G.A. (2015). Amyloid properties of the mouse egg zona pellucida. PLoS One. 2015 Jun 4;10(6):e0129907. doi: 10.1371/journal.pone.0129907. eCollection 2015. PubMed
- Guyonnet B., Egge N., and Cornwall, G.A. (2014). Functional amyloids in the mouse sperm acrosome. Mol Cell Biol. 34:2624-34. PubMed
- Whelly, S., Serobian G., Borchardt C., Powell, J., Johnson, S., Hakansson, K., Lindstrom, V., Abrahamson, M., Grubb, A., and Cornwall, G.A. (2014). Fertility defects in mice expressing the L68Q variant of human cystatin C:a role for amyloid in male infertility. J Biol Chem 14;289:7718-29. PubMed
- Guyonnet, B., Zabet-Moghaddam, M., SanFrancisco, S., and Cornwall, G.A. (2012). Isolation and proteomic characterization of the mouse sperm acrosomal matrix. Mol Cell Proteomics 11:758-774. PubMed
- Whelly, S., Johnson, S.S., Powell, J., Borchardt C., Hastert, M-C, and Cornwall, G.A. (2012). Nonpathological extracellular amyloid is present during normal epididymal sperm maturation. Plos One, 10.1371/journal.pone.0036394. PubMed
- Tardif S., Guyonnet, B., Cormier, N., and Cornwall, G.A. (2012). Alteration in the processing of the ACRBP/sp32 protein and sperm head/acrosome
malformations in proprotein convertase 4 (PCSK4) null mice. Mol Human Reprod. 18:298-307.
Cover photo PubMed - Chau, K. and Cornwall, G.A. (2011). Impaired fertility in vitro in mice lacking the cystatin CRES (cystatin-related epididymal spermatogenic): rescue by exposure of spermatozoa to dibutyryl cAMP/IBMX Biol Reprod. 84:140-52. PubMed
- Parent, A.D., Cornwall, G.A., Liu, L.Y., Smith, C.E., and Hermo, L. (2011). Alterations in the testis and epididymis associated with loss of function of the cystatin related epididymal spermatogenic (CRES) protein. J Androl 32; 444-63. PubMed
