Roger B. Sutton, Ph.D.
Professor of Cell Physiology and Molecular Biophysics
Texas Tech University Health Sciences Center
3601 4th Street, STOP 6551
Lubbock, Texas 79430
Office Phone: (806) 743-4058
Office room: 5A171A
E-mail:roger.b.sutton@ttuhsc.edu
Research Interest:
X-ray crystallography, membrane fusion, C2 domain biophysics (synaptotagmin and dysferlin)
Our lab uses LabGuru
Full list of publications: PubMed
Sutton Lab:
Our lab specializes in the 3D structure of C2 domains.
Synaptotagmin
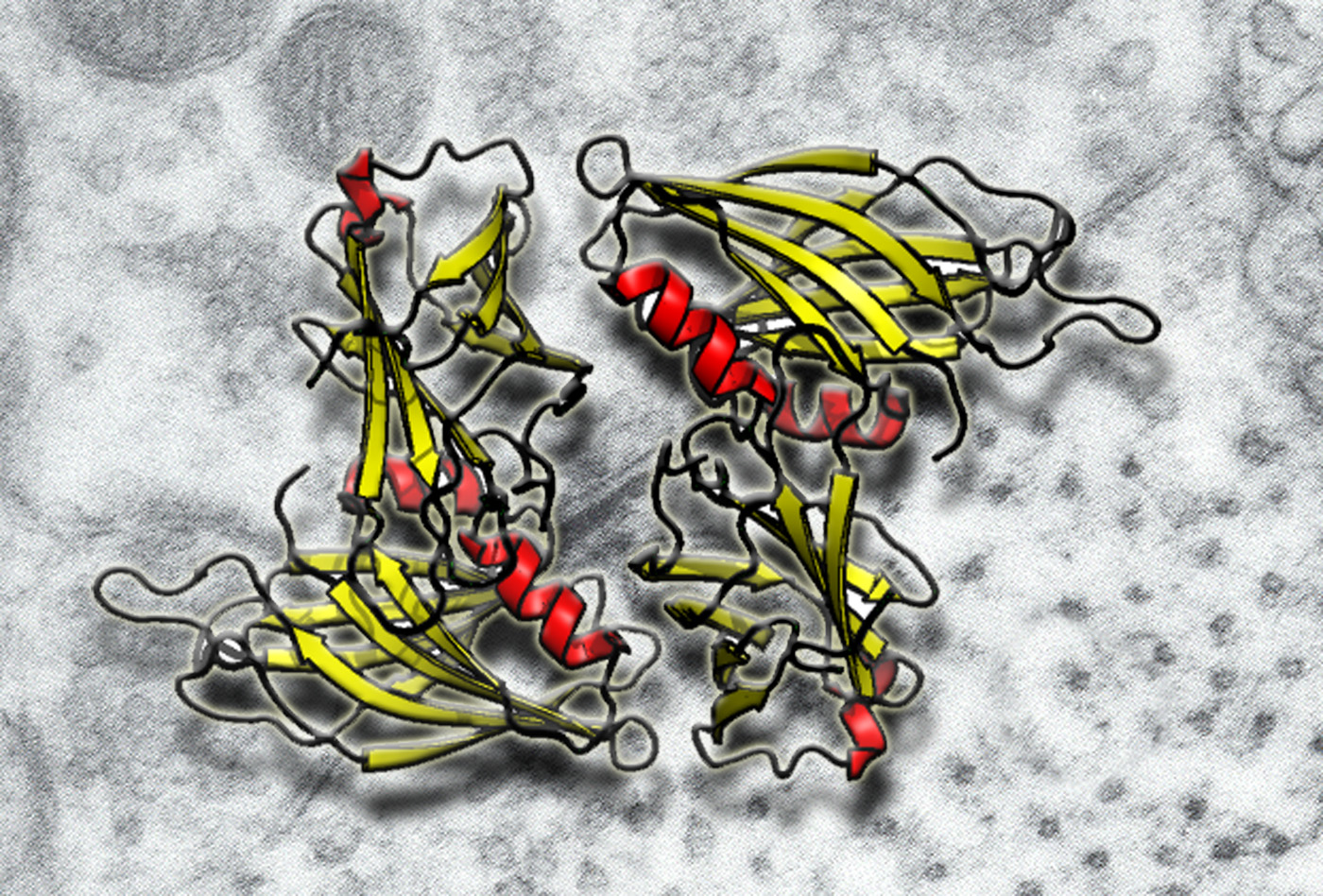
Synaptotagmin is the Ca2+ sensor for exocytosis. We have crystallizes the two tandem C2 domains of human synaptotagmin. This crystal structure shows clear signs of inter-domain interaction.
Fuson KL, Montes M, Robert JJ, Sutton RB. Structure of human synaptotagmin 1 C2AB in the absence of Ca2+ reveals a novel domain association. Biochemistry. 2007 Nov 13;46(45):13041-8. Epub 2007 Oct 23.
Dysferlin
Muscle is unique among the tissues of the body in that its primary function is to generate mechanical force. These relatively large forces introduce damage in the form of rips and tears within the membrane of the muscle cells. Without an in vivo repair mechanism, muscle tissue would be unable to function. Dysferlin is a 237kDa multi-domain protein that is expressed at high levels in skeletal and cardiac muscle tissue, and appears to play a critical role in resealing tears in the muscle cell phospholipid membranes. This protein is made up of 7 tandem C2 domains (labeled C2A, C2B, C2C...) that are distributed along the length of the protein. Mutations within the dysferlin gene in humans can result in Limb-Girdle muscular dystrophy (LGMD), presumably resulting from an impaired ability to reseal damaged muscle tissue. To understand what this protein could be doing at the atomic level, we recently solved the 2.0Å X-ray crystal structure of the C2A domain of human dysferlin.
Now that we have a high resolution structure of one of these domains, we would like to establish why point mutations such as V67D produce severe phenotypes in Limb-Girdle Muscular Dystrophy patients. We hope to one day discover drugs that may stabilize this mutant C2 domain, to provide some degree of treatment for patients with this form of muscular dystrophy.
Got Questions?
We're here to help. Contact us if you have questions.

