Robert Bright, PhD

Robert Bright, Ph.D.
Professor
Office Phone: 806-743-4592
E-mail: Robert.Bright@ttuhsc.edu
Education:
IRTA Fellow: National Cancer Institute, National Institutes of Health (1996)
PhD: University of Texas Health Sciences Center, San Antonio (1994)
BS: University of Idaho (1987)
Biography:
Robert K. Bright is a Professor and Graduate Program Advisor for the Department of
Immunology and Molecular Microbiology at Texas Tech University Health Sciences Center
in Lubbock, Texas. Dr. Bright graduated from the University of Idaho in Moscow, Idaho
with a B.S. degree in Bacteriology (emphasis biochemistry) in 1987. After some time
on active duty as an officer in the U.S. Army he was hired as a Research Associate
at the SW Foundation for Biomedical Research in San Antonio, TX. Dr. Bright enrolled
in the graduate program at the University of Texas Health Sciences Center at San Antonio
in the fall of 1989. He was recalled to active duty in the U.S. Army in the fall of
1990 and served until June of 1991 in the combat zone in support of Operation Desert
Storm. Returning to graduate studies in the fall of 1991 Dr. Bright earned a Ph.D.
in Microbiology and Immunology from the Department of Microbiology and Immunology
at the University of Texas Health Sciences Center in San Antonio in 1994. Upon completion
of his doctoral studies Dr. Bright moved to Bethesda, Maryland where he was awarded
an Intramural Research Training Award Fellowship to study tumor immunology in the
Surgery Branch of the National Cancer Institute, National Institutes of Health. In
late 1996 he was recruited to the Karmanos Cancer Institute and the Department of
Surgery at the Wayne State University School of Medicine in Detroit, Michigan where
he assumed a position of Assistant Professor. Two years later Dr. Bright was recruited
to the Earle A. Chiles Research Institute and the Robert Franz Cancer Center at the
Providence Portland Medical Center in Portland, Oregon where he served as Chief of
Prostate Cancer Biology until February Center Graduate School of Biomedical Sciences
Outstanding Teacher and Student Advocate Award, 2006. He was recognized, in 2011,
by the DOD Prostate Cancer Research Program as an Innovative Mind in Prostate Cancer
Research for his work on cancer vaccines. Dr. Bright has served and currently serves
on numerous national grant review committees to include the American Cancer Society,
the DOD/ Congressionally Directed Medical Research Program, NASA and the National
Institutes of Health. He has also served as Chair for several grant review committees.
Dr. Bright also serves as an expert reviewer for several scientific journals including
the Journal of Immunology, Cancer Immunology and Immunotherapy, the Journal of Immunotherapy,
Vaccine, Cancer Research, Clinical Cancer Research. He currently serves as an Associate
Editor for the journal ISRN Immunology. His research focuses on tumor immunity with
emphasis on the development of vaccines against cancer and has been funded by the
National Cancer Institute/NIH, the American Cancer Society and the DOD CDMRP Prostate
Cancer Research Program. Dr. Bright has published over 50 manuscripts and several
book chapters and reviews on tumor immunology and cancer vaccines. of 2002. In 2002
Dr. Bright was recruited as an Associated Professor to the Department of Immunology
and Molecular Microbiology at Texas Tech University Health Sciences Center. Dr. Bright
has received several honors and awards to include the University of Texas Health Sciences
Center Armand J. Guarino Award for Academic Excellence in Doctoral Studies (top graduate
student), the National Institutes of Health Fellows Award for Research Excellence
(top 10% of postdoctoral fellows), a Research Scholars Award from the American Cancer
Society and the Texas Tech University Health Sciences Center.
Research Interests:
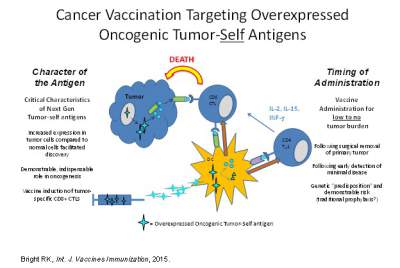
Extensive studies over the past few decades have established that cancer patients
can mount immune responses against their own tumor(s), and that these responses
can be effectively manipulated to treat patients with cancer by using defined targets
isolated from the tumor cells, known as tumor associated antigens as vaccines. Recently
the National Cancer Institute sponsored a pilot project to prioritize cancer vaccine
target antigens for translational research. The study involved developing a set of
nine “ideal” cancer antigen criteria/ characteristics, with 1) therapeutic function,
2) immunogenicity and 3) a role in oncogenicity deemed as having the greatest importance.
From a growing but representative list of antigens, a group defined as over-expressed
self-proteins stands out as the group with the largest number of potentially important
target antigens. My research at present has focused on tumor immunology and cancer
vaccines, which can be categorized by work in: a) the discovery and validation of
novel tumor associated antigens using genomics and proteomics, b) the design of
vaccine strategies and c) the study of mechanisms that impede effective vaccine
induced immunity to include CD4+ and CD8+ regulatory T cells. Our systems biology
efforts along these lines have led to the discovery of a novel cancer over-expressed
self onco-protein, Tumor Protein D52, which is an active focus of our research.
Tumor protein D52 (D52) is involved in cellular transformation, proliferation and
metastasis and thus has a role in oncogenicity. Expression microarray analysis predict
D52 over-expression in many other cancer types, including multiple myeloma, Burkitt’s
lymphoma, pancreatic cancer, testicular germ cell tumors, and melanoma. We have reported
that vaccines targeting D52 elicit tumor-specific T cells but only limited tumor
protection in murine models of cancer. Data from these D52 vaccine studies suggest
that a subset of CD8+ T cells plays a regulatory role in suppressing vaccine induced
D52-specific immunity and may play a role suppressing immunity to self proteins
in general. A deeper understanding of the regulation of immunity to self onco-proteins
is critical for the development of successful cancer vaccines. Our goal is to develop
D52 as a systemic therapy or vaccine against cancer. Since D52 is over-expressed
in many malignancies the long-term clinical impact could be wide reaching.
Current and Former Students and Postdocs:
Graduate Students:
- Revathi Govind, Ph.D., 2005. Microbial Genetics, Microbiology and Immunology, TTUHSC, Lubbock, TX.
- Enusha Karunasena, Ph.D., 2005. Microbial Genetics, Microbiology and Immunology, TTUHSC, Lubbock, TX.
- Colby Layton, Ph.D., 2006. Microbial Pathogenesis, Microbiology and Immunology, TTUHSC, Lubbock, TX.
- Landon Westfall, Ph.D., 2006. Microbial Pathogenesis, Microbiology
 and Immunology, TTUHSC, Lubbock, TX.
and Immunology, TTUHSC, Lubbock, TX. - James Schaber, Ph.D., 2006. Microbial Pathogenesis, Microbiology and Immunology, TTUHSC, Lubbock, TX.
- Matthew R. Fogle, Ph.D. 2007, Microbial Pathogenesis, Microbiology and Immunology, TTUHSC, Lubbock, TX.
- Summer Ketron-Adkins, M.S. 2008, Immunology, Biology, TTU,. Dental student UTHSC, Houston, TX.
- Mona Shehata, Ph.D., 2007. Cancer Genetics, Molecular Oncology Laboratory, Oncology Research Unit, Children’s Hospital at Westmead, Sydney, Australia. Fellow, London, England.
- Devin B. Lowe, Ph.D. 2010, Tumor Immunology, Microbiology and Immunology, TTUHSC, Lubbock, TX. Adjunct Assistant Professor, TTUHSC, Abilene.
- Heather Decker, M.S., 2010, Tumor Immunology, Microbiology and Immunology, TTUHSC, Lubbock, TX.
- Joel Aldrich, summer 2015, TTU. Ph.D.; 2012, Tumor Immunology, Microbiology and Immunology, TTUHSC, Lubbock, TX. Earned JD (co-Mentored with Dr. Ronald Kennedy).
- Gurvinder Kaur, Ph.D., 2012, Transplantation T1D, Cell Biology and Biochemistry, TTUHSC, Lubbock, TX. Post-doc fellow, Cell Biology and Biochemistry, TTUHSC, Lubbock TX.
- Eric Veggeberg, M.S., 2012, Tumor Immunology, Microbiology and Immunology, TTUHSC, Lubbock, TX.
- Rosabel Loya, M.S., 2012, Tumor Immunology, Microbiology and Immunology, TTUHSC, Lubbock, TX. Podiatry School, Miami, FL.
- Bhagavathi Ramasubramanian, Ph.D., 2012. Microbial Genetics, Microbiology and Immunology, TTUHSC, Lubbock, TX.
- Payal Mital, Ph.D., 2013. Transplantation T1D, Cell Biology and Biochemistry, TTUHSC, Lubbock, TX. Post-doc fellow, Pennsylvania.
- Jake Everett, Ph.D. 2015, Microbial Pathogenesis, Immunology and Molecular Microbiology, TTUHSC, Lubbock, TX.
- Somnath Pandey, Ph.D. 2016, Cancer Epigenetics, Immunology and Molecular Microbiology, TTUHSC, Lubbock, TX
- Cynthia Reinoso-Webb, Ph.D. 2017, Inflammatory Bowel Disease, Immunology and Molecular Microbiology, TTUHSC, Lubbock, TX
- Kandis Wright, M.D., Ph.D. candidate, Cell Biology and Biochemistry, TTUHSC, Lubbock, TX
- C. Riccay Elizondo, Ph.D. candidate, Immunology & Molecular Microbiology, TTUHSC, Lubbock, TX.
Postdoctoral Fellows, Research Associates, Residents, and Fellows:
- Eric T. Kimchi, M.D., 1998-1999. Present Position: Faculty, Surgery, University of Chicago, Chicago, IL.
- Libang Yang, M.D., Ph.D., 2000-2002. Present Position: Senior Associate, University of Minnesota Cancer Center, Minneapolis, MN.
- Rui Li, PhD. 2000-2002. Present Position: Senior Research Associate, Franz Cancer Center, Portland, OR.
- Jason Hurt, MD. Spring 2005-2008. Medical Director-Oncology, Medpace, Cincinnati, OH.
Medical Students:
- Stephanie Villareal, MD student, June-August, 2010. Medical Student Summer Research Program
- Jaime Camocho, MD student. June- August, 2015. Medical Student Summer Research Program.
Undergraduate Students, High School Students, and Other Individuals:
- Shahana Baig, 1999-2001. 40 hours per week during summer. Graduated Medical School.
- Matthew Lewis, 2001. 40 hours per week during summer. Graduated Medical School.
Grace, Tarng, June 2003- September 2003, 10-15 hrs/ week, HHMI research fellow, TTU. - Dung Nguyen, June 2005- August 2005, 15- 20 hrs/week, JAMP, (UT, Austin)
- Devin Lowe, June 2003-2005, HHMI research fellow (TTU), TTU Honors College, Co-advisor on Devin’s thesis and thesis work. Ph.D., 2010, TTUHSC. Adjunct Assistant Professor, TTUHSC, Abilene, TX.
- Laura A. Payton, 2003-2006. 20 hours per week, 12 months per year. Cancer Vaccine Strategies in Murine Tumor Models (Thesis Advisor). HHMI research fellow, TTU, TTU Honors College. Ph.D., 2010, UT Southwestern, Dallas, TX. Expert Consultant, Boston, MA.; Senior Scientist Genentech San Francisco,CA
- Adam Wolfe, 2007-2009. Proteomics-based Identification of Novel Targets in Cancer.
HHMI research fellow, TTU. Doctoral (MD/PhD) Candidate at UTHSC, Houston, TX, June
2009-present.
Emily Powell, 2010-2011. 20 hours per week, 12 months per year. Assessing the Role of TPD52 in Tumor Cells with RNAi-Based Expression Analysis . HHMI research fellow, TTU. Ph.D. candidate, Chemistry, UT at Austin. - Ksenija Korac, 2016-2017. Molecular Analysis of T cell responses to the neo-TAA TPD52. TTU Biochemistry, Lubbock TX.
Selected Publications:
- Bright RK. (2023) Preclinical support for tumor protein D52 as a cancer vaccine antigen. Hum Vaccin Immunother 19:2273699. PMID: 37904517.
- Washburn RL, Martinez-Marin D, Sniegowski T, Korac K, Rodriquez AR, Miranda JM, Chilton BS, Bright RK, Pruitt K, Bhutia YD, Dufour JM. (2023) Sertoli Cells Express Accommodation, Survival and Immunoregulatory Factors When Exposed to Normal Human Serum. Biomedicines 11:16501670.
- Elizondo CR, Bright JD, Bright RK (2022). Vaccination with a Shared Oncogenic Tumor-self Antigen Elicits CD8+ T cells with a Regulatory Phenotype. Hum Vaccin Immunother, 18:(e2108656) Impact Factor:3.643
- Castro-Piedras I, Sharma M, Brelsfoard J, Vartak D, Martinez EG, Rivera C, Molehin D, Bright RK, Fokar M, Guindon J and Pruitt K. (2021) Nuclear Dishevelled targets gene regulatory regions and promotes tumor growth. EMBOJ, 22: e50600. Impact factor: 8.65
- Elizondo CR, Bright JD Byrne JA and Bright RK (2020). Analysis of CD8+ IL-10+ T cells elicited by vaccination with the oncogenic tumor-self protein D52. Hum Vaccs and Immunother 16:1413-1423. Impact Factor: 3.643
- Hong Yu, Jonathan Arthur, Yuyan Chen, Guy Groblewski, Laurence Cantrill, Sarah Frost, Robert K Bright, Matloob Khushi and Jennifer A Byrne. (2019). Delayed recruiting of TPD52 to lipid droplets - evidence for a "second wave" of lipid droplet-associated proteins that respond to altered lipid storage induced by Brefeldin A treatment. Scientific Reports 9(9790): 1-21. Impact Factor: 4.525
- Dong Z, Yu D, Liu Q, Ding Z Lyons VJ, Bright RK, Pappas D, Liu X and Li W. (2018). Enhanced capture and release of circulating tumor cells using hollow glass microspheres with a nanostructured surface. Nanoscale. 10(35):16795-16804. Citations: 20, Impact Factor: 6.970
- Bright RK. (2017). Arming the Immune System for the War on Cancer. Research Features. 114:16-19. http://cdn.researchfeatures.com/3d_issues/issue114/html5/index.html
- Bright RK. (2016). Cancer Vaccines: The next generation immunotherapy. Int. J. Vaccines and Immunization Feb. 2(1): https://www.sciforschenonline.org/journals/vaccines/article-data/IJVI-2-105/IJVI-2-105.pdf
- Bright RK and Mamula MJ. (2015). Regulation of adaptive immune responses to self-antigens in cancer and autoimmunity. Curr Trends Immunol. 15:47-57.
- Bright RK, Bright JD and Byrne JA. (2014). Overexpressed Oncogenic Tumor-Self Antigens:
New Vaccine Targets. Human Vaccines and Immunotherapeutics, 10:3297-305.
Byrne JA, Frost S, Chen Y, and Bright RK. (2014).Cancer Biology: Tumor protein D52 (TPD52 ) and cancer: oncogene understudy, or understudied oncogene? Tumour Biol 35:7369-82,2014. - Bright JD, Aldrich JF, Byrne JA and Bright RK. (2014). Vaccination with the Prostate Cancer Over-Expressed Tumor Self-Protein TPD52 Elicits Protective Tumor Immunity and a Potentially Unique Subset of CD8+ T Cells Austin J Clin Immunol.1(2):1-13. http://www.austinpublishinggroup.com/clinical-immunology/fulltext/ajci-v1-id1007.php
- Bright JD, Schultz HN, Byrne JA and Bright RK. (2013). Injection Site and Regulatory T Cells Influence Durable Vaccine-Induced Tumor Immunity to an Over-Expressed Self Tumor Associated Antigen. OncoImmunology 7(2):(e25049) 1- 11.
